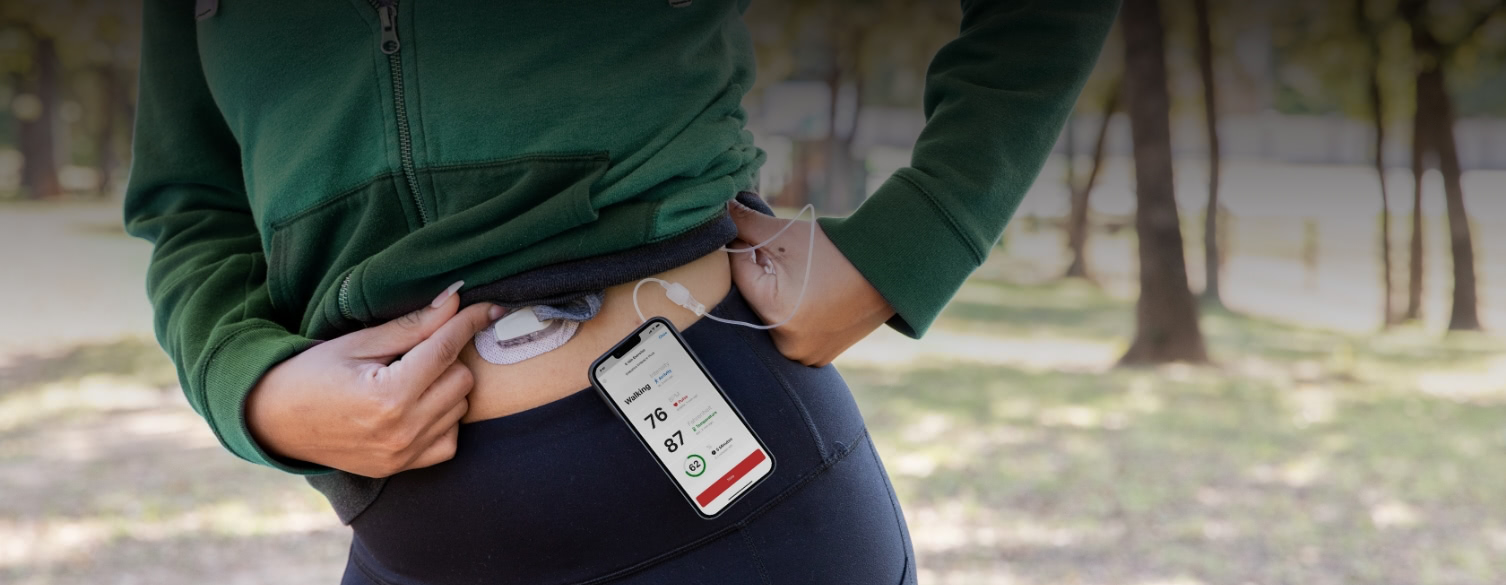


Knowledge and expertise gained from thousands of clinical trials – combined with our flexible, validated, and regulatory-compliant technology – allows PDH to quickly capture your requirements and deliver seamlessly integrated clinical workflows for even the most complicated trial designs.
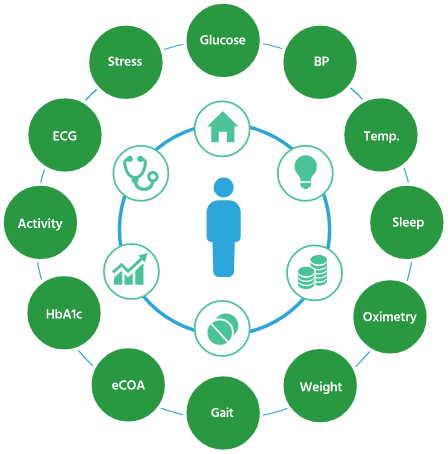


SUMMA™ is a flexible, modular, and fully-validated technology platform that accommodates a wide range of study designs, devices, and vendors. Clinical experts helped design its unique architecture, allowing it to be easily configured to suit the parameters of your next trial with full regulatory compliance.

Sponsors and CROs trust PDH to solve problems that keep complex trials from running smoothly, pushing the boundaries of what’s possible for today’s rapidly changing digital and decentralized trial landscape. We’re creative, specialized, and a little obsessed—which is why we’re able to build such strong customer relationships across the industry.

MAIN OFFICE
15615 Alton Parkway,
Suite 450
Irvine, California 92618

